Oceans in Peril: The Other Danger of CO2
Oceans in Peril, a cry of alarm resonates in the face of the growing threat looming over these blue expanses, sources of life and lungs of our planet. Far from the spotlight on global warming, another danger, insidious and just as ruthless, gnaws at the depths: ocean acidification.
The culprit? Carbon dioxide (CO2), the greenhouse gas relentlessly released into the atmosphere by human activities. Like a stealthy poison, it dissolves into the marine waters, causing a chemical reaction with dramatic consequences.
The Ocean as a CO2 Sink
Before the industrial revolution, the ocean was a source of CO2 in the atmosphere and balanced the impact of rock weathering and terrestrial particulate organic carbon. But nowadays, the ocean has become a sink for excess atmospheric carbon dioxide.
By absorbing about 30% of the anthropogenic atmospheric CO2 produced by humans, oceans play a crucial role in climate regulation. This reduction in climate change impact occurs through both physical and biological processes.
Physical Process
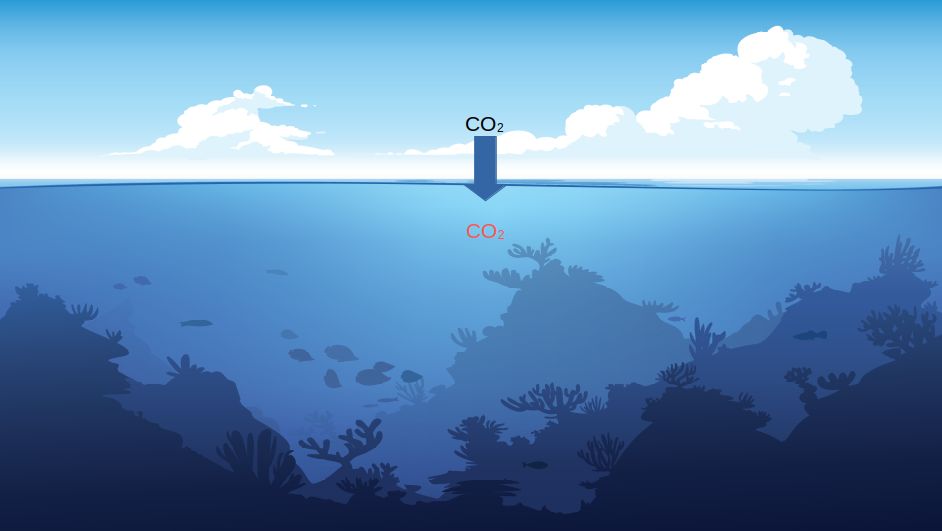
Atmospheric CO2 naturally dissolves into the oceans at the air-water interface. The more CO2 in the atmosphere, the more it will dissolve into the oceans.
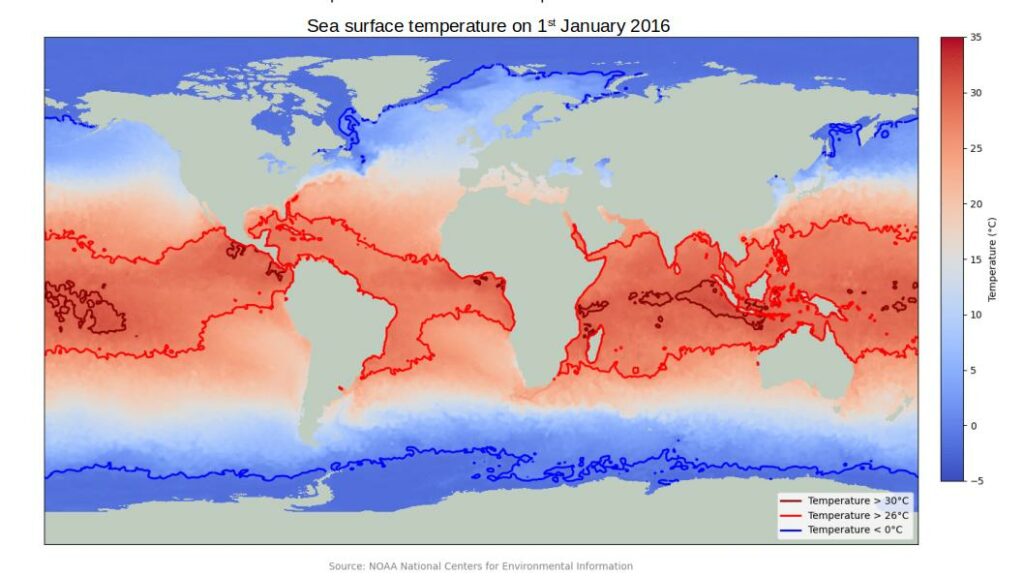
This dissolution is favored at lower temperatures. Thus, colder oceans absorb more CO2 than warmer oceans. This translates globally to less absorption at the equator and more absorption of CO2 closer to the poles.
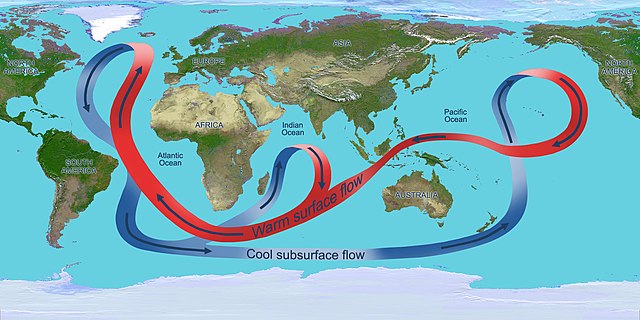
The thermohaline circulation carries equatorial waters towards the poles, resulting in cooling of the waters, making them colder, denser, and more CO2 concentrated. These waters eventually sink, carrying CO2 to the ocean depths. They are replaced by warmer water from the equator, less CO2 concentrated, which will, in turn, cool down, absorb CO2, and eventually sink into the depths.
Biological Process
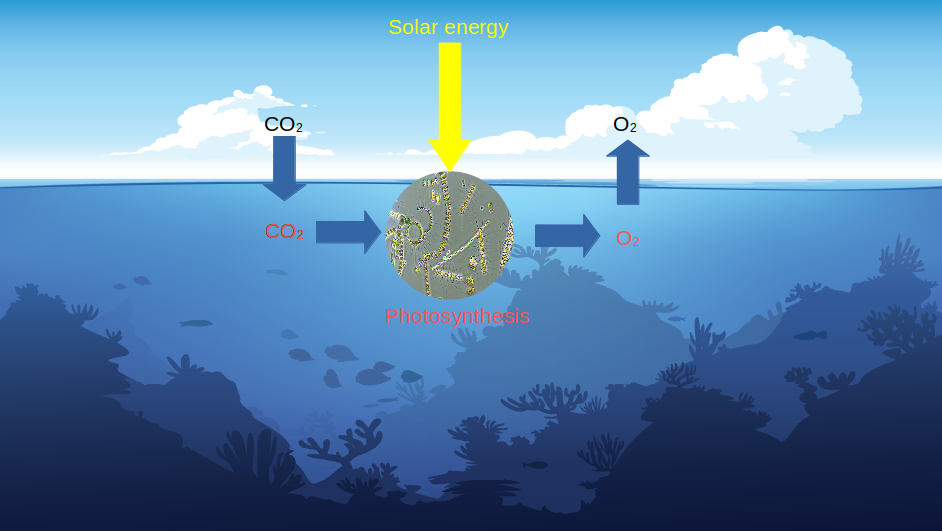
The absorption of atmospheric CO2 also occurs through photosynthesis. In this case, microscopic algae called phytoplankton, located at the ocean surface, absorb CO2 from the atmosphere and transform it into oxygen (O2) using sunlight, according to the following reaction:

Photosynthesis is accompanied by the transformation of inorganic carbon (CO2) into organic carbon.
The biological process is useful from two perspectives: firstly, it produces oxygen for life (the ocean being the planet’s main lung), and secondly, it removes some of the CO2 from the atmosphere because when phytoplankton dies, it sediments to the ocean floor and no longer contributes to the greenhouse effect.
The Ocean as Efficient as Forests
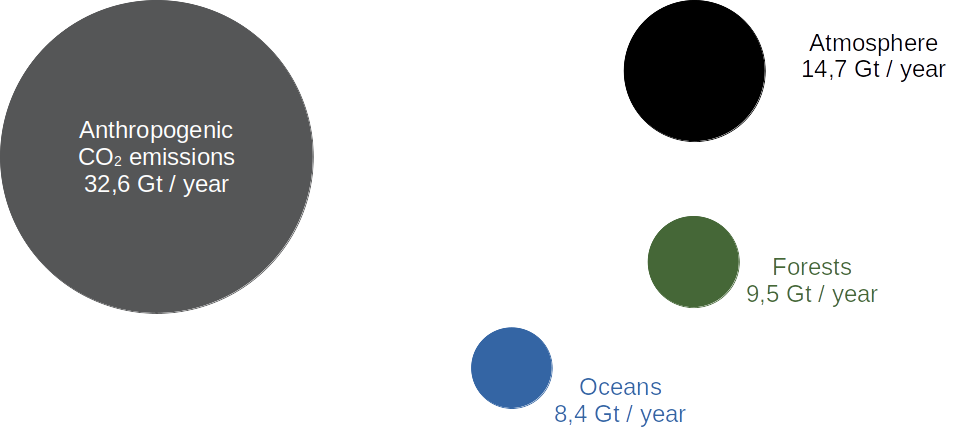
Thanks to these biological and physical processes, the ocean helps reduce the accumulation of atmospheric CO2 and thus its impact on climate change, while also facilitating the creation of oxygen necessary for life on Earth. Forests are often referred to as the planet’s “green lung.” The ocean can literally be called the “blue lung” of our Earth because it is just as efficient as forests as a carbon sink. For every 32.6 gigatons (Gt) of anthropogenic CO2 emitted each year, 8.4 Gt are absorbed by the oceans, 9.5 Gt by forests, and 14.4 Gt remain in the atmosphere.
Causes of Ocean Acidification
This blue lung seems to be a boon for the planet in its fight against CO2 emissions-related global warming, but as CO2 dissolves in the ocean, it alters marine chemistry.
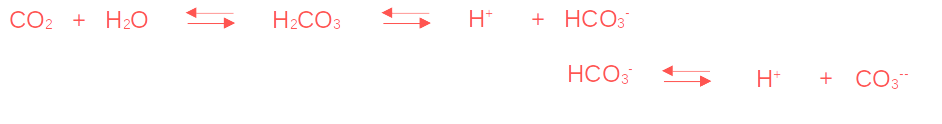
Indeed, when CO2 dissolves in water, it forms carbonic acid (H2CO3). This carbonic acid dissociates into hydrogen ions (H+) and bicarbonate ions (HCO3–). When the second hydrogen atom of carbonic acid is released, bicarbonate (HCO3–) transforms into carbonate (CO3—).
The result of ocean CO2 absorption is an increase in the number of hydrogen ions in the oceans, leading to a decrease in pH and hence an increase in acidity. This pH variation is buffered by carbonate ions (CO3—), which limits significant acidity changes despite the dissolution of significant amounts of CO2. The amount of carbonate ions in the oceans thus corresponds to the limit of its buffering capacity. The risk is therefore to see significant changes in acidity when carbonate ions can no longer counterbalance pH.
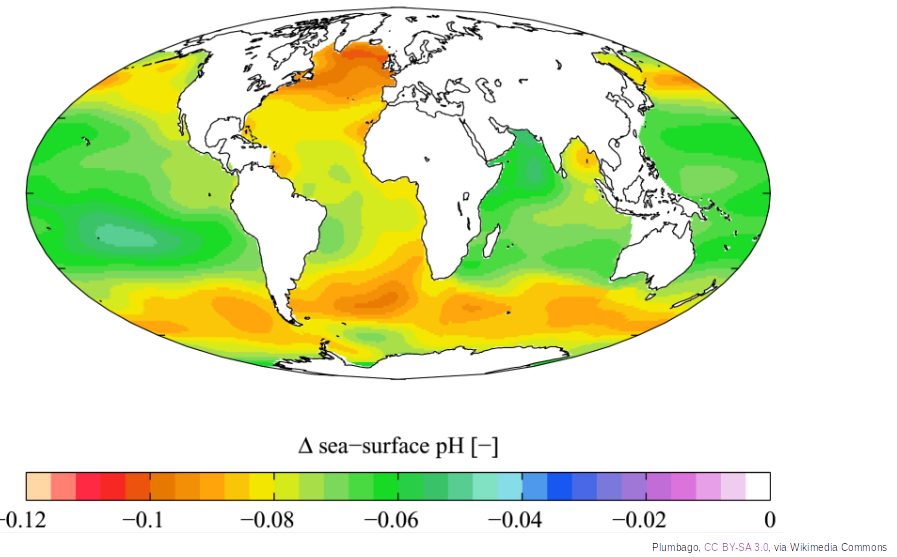
The ocean is slightly alkaline with an average pH of about 8.1, but it was about 8.2 before the industrial revolution, which corresponds to a 30% increase in ocean acidity compared to the pre-industrial period. According to the sixth IPCC report, ocean pH could drop to 7.8 by 2100, corresponding to a 150% increase in acidity. This significant pH variation would have a detrimental impact on aquatic life.
Impacts of Ocean Acidification on Marine Life
The ocean’s buffering capacity is essentially due to carbonate ions. The more CO2 is dissolved in the ocean, the more carbonate (CO3—) transforms into bicarbonate (HCO3–). This reaction leads to a decrease in carbonate ions availability (CO3—). However, calcifying organisms, such as oysters, crabs, sea urchins, lobsters, corals, and even phytoplankton, require carbonate (CO3—) to develop and maintain their shells and skeletons.

Moreover, the increase in ocean acidity causes the shells and skeletons of these organisms to decompose more easily. Eventually, there is a real risk that ocean water becomes corrosive to these organisms, leading to the disappearance of carbonate-shell/skeleton-containing species. The impact on ecosystems and biodiversity would be catastrophic. At this stage, it would represent a mass extinction. A point of no return would be reached for marine life, but also for coastal populations and ecosystems heavily dependent on the marine environment.
Ocean, CO2, and Climate Change: The Vicious Circle
With global warming, the oceans warm up, and as previously discussed, the higher the ocean temperature, the less it can absorb CO2. This corresponds to a vicious circle because the more CO2 accumulates in the atmosphere, the more it will further accentuate the planet’s warming.
In addition to this CO2 aspect, the ocean acts as Earth’s thermostat by regulating the planet’s temperature by storing and redistributing heat. Disruption of ocean temperatures may also exacerbate climate change.
Let us not forget also that with ocean acidification, there is a real risk of it becoming corrosive and part of the phytoplankton disappearing. With them, the disappearance of photosynthesis, which absorbs atmospheric CO2, with the release of oxygen (O2) necessary for life.
The ocean continues to absorb CO2, but with decreasing effectiveness. CO2 is stored less and less deeply. If humans were to completely stop CO2 emissions, it would take 1000 years for the oceans to absorb 80% of the anthropogenic CO2 already emitted.
Oceanographers have, however, noticed cycles in ocean CO2 levels, with periods when the ocean absorbs more CO2 and others when it absorbs less. Scientists are currently trying to understand these cycles to better understand future changes in oceanic absorption of anthropogenic CO2. But as long as CO2 is emitted into the atmosphere, the oceans will continue to absorb and become acidic.
Solutions to Protect Oceans and Marine Ecosystems
For the past 200 years (since the industrial revolution), the Earth has undergone significant climate change and ocean acidification. Ocean acidity has increased by 30% since the industrial revolution, and forecasts indicate a 150% increase in ocean acidity by 2100.
Currently, the ocean helps absorb a portion of the atmospheric CO2 emitted by human activity, thereby assisting in reducing the impact on climate change. However, given current developments and the physico-chemical impact on the oceans, the ocean’s capacity to absorb CO2 is diminishing, which will ultimately lead to a more significant impact on climate change and the oceans.
Unfortunately, the only way to break out of this vicious cycle and avoid mass extinction is to reduce CO2 emissions by developing non-CO2-emitting energy sources.